Products and solutions
At Mölnlycke, we are dedicated to helping you, help patients. Explore our range of innovative medical products and solutions.
Surgical

Staff clothing
Discover the BARRIER® range of staff clothing – a wide range of protective clothing for...

Biogel surgical gloves
Discover the proven quality of Biogel® surgical gloves – reliable protection for your hands...
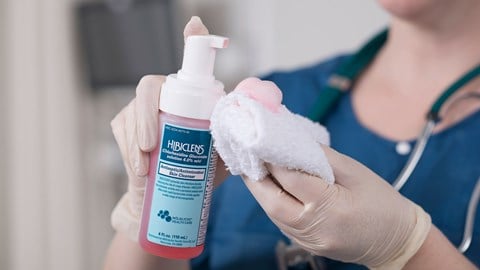
Hibi Antiseptics
Discover our range of antiseptic products
Wound care

Mepilex Foam dressings
Discover the Mepilex® range of wound treatment and prevention solutions.

Mepitel dressings
Mepitel® is a gentle, yet effective wound contact layer with the unique Safetac® technology.

Exufiber dressings
Exufiber® - gelling fiber dressings, featuring Hydrolock® Technology.

Wound management
Discover our wound management solutions – all designed to help wounds heal.
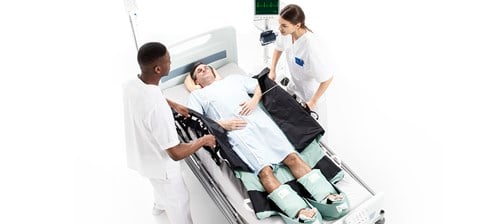
Turning and Positioning system
Products specifically designed pressure ulcer prevention

